When it comes to flammable gases, we speak of naturally occurring gases (e.g. methane), flammable pure substances (e.g. hydrogen), and evaporation products from flammable and volatile substances or liquids (e.g. acetone).
When working with flammable and explosive gases it is vitally important to know the working limits of those substances. This is why you should always read the accompanying Safety Data Sheets on products that you will work with, to stay informed about the risks.
In this article, we discuss the characteristics of the following flammable gases: Ammonia, Acetylene, Butane, Carbon Monoxide, Hydrogen, Methane, Propane, Ethane, Ethylene, Silane and Chlorine Trifluoride.
Official Definitions of Gases and Vapors
By definition, according to the Globally Harmonized System of Classification and Labelling of Chemicals (GHS): A Flammable gas is a gas that has a flammable range at STP (Standard Temperature and Pressure) of 20℃ and 101.3 kPa. These are considered normal conditions.
- “Gases” refers to materials that are in a gaseous state under normal atmospheric conditions. Examples of this would be hydrogen or methane.
- “Vapors” refers to the gases over a material that is a liquid under normal atmospheric conditions but emits gases within the flammable range under these atmospheric conditions.
Inert Gases, Oxidizers and Flammable Gases
Gases are typically categorized into three distinct groups:
- Oxidizers
- Inert gases
- Flammable gases
Something that is inert simply doesn’t react with other things, such as the noble gases helium, argon, krypton, neon, and radon. Another well-known inert gas is carbon dioxide.
Oxygen is not flammable, nor is bromine gas, but both are oxidizing agents and support vigorous combustion. Bromine, the only liquid non-metallic element, doesn’t become gas until 58℃, which never normally happens on Earth’s surface where the highest recorded temperature was 56.7℃ in Death Valley in 1913.
With this understanding of oxidants and inert gases, let’s now focus on the main topic: flammable gases.
Flammability Limits – When Does a Flammable Gas Ignite?
What Are LEL & UEL?
When a flammable gas mixes with oxygen in the correct proportions and encounters an ignition source, it can lead to explosions or fires.
Many consumer electronics have the potential to produce electric arcs, which is why they can become sources of ignition. This is the reason operators in hazardous areas must use specialized equipment, such as our intrinsically safe camera.
The Lower Explosion Limit (LEL) and Upper Explosion Limit (UEL) are much as you imagine—below LEL or above UEL an otherwise combustible gas does not form an explosion hazard. For example, hydrogen gas has a very broad range and creates an explosive atmosphere when the concentration in the air exceeds 4% while remaining below 76%.
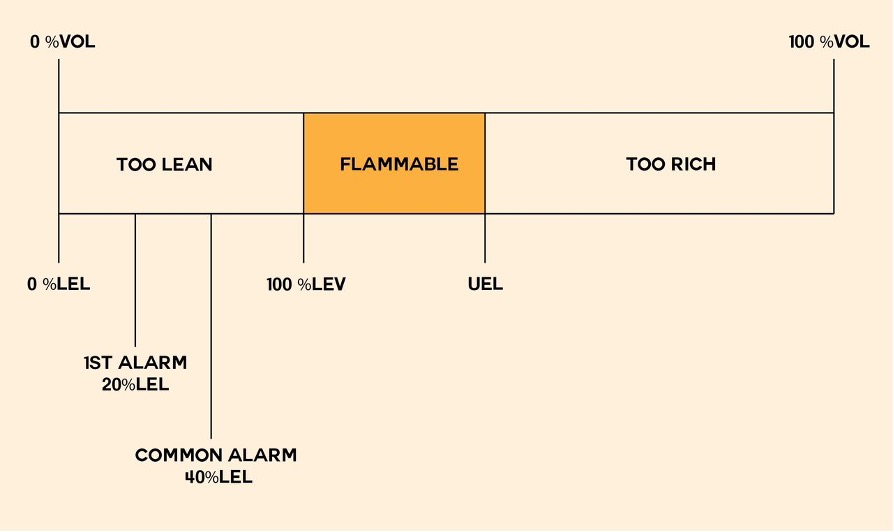
Flammability Limits vs. Explosion Limits
The lower flammability limit of a specific flammable gas determines the minimum concentration of the gas mixed with air required to form a flammable mixture. In other words, only when a flammable gas is mixed with oxygen in the right proportions can it ignite and start a fire. However, it’s important to note that a mixture of flammable gas and air doesn’t necessarily always lead to combustion.
For many substances, the flammability limits and explosion limits are identical, which is why people often use these terms interchangeably.
With that in mind, people often ask how more than 60% of passengers survived the 200-foot (61-meter) drop and crash of the hydrogen-filled dirigible Hindenburg in 1937. Simply put, the leaking gas ignited as it rose and met with air above the passengers, taking much of the heat with it.
Yes, the silken and rubberized material of the envelope burned, too, but the gas itself was too concentrated to explode, per se, burning at the interface where it met the air. If it had exploded, instead of just burning, there would have been no survivors.

[Table] LEL & UEL For Various Combustible Gases
Of course, the concentrations required to reach these dangerous levels vary with the different gases involved. Concentrations below the LEL are too ‘lean’ to explode; concentrations above the UEL are too ‘rich’. Let’s look at some of the most common gases:
| Gas (and vapors) | LEL % | UEL % | Comment |
|---|---|---|---|
| Acetone | 2.2 | 13 | Common nail polish remover. |
| Acetylene | 2.5 | 100* | *Catalyses with some metals and can explosively decompose without oxygen present at pressures >15 psi. |
| Ammonia | 15 | 28 | Fortunately, its intrinsic odor keeps you from experiencing significant, toxic, or dangerous concentrations. |
| Butane | 1.8 | 8.4 | Butane is frequently used as portable cooking fuel. |
| Carbon Monoxide | 12.5 | 74 | By-product of combustion. |
| Dimethyl Ether | 3.4 | 27 | Synthetic diesel fuel replacement. |
| Diethyl Ether | 1.7 | 36 | Too inflammable for anesthesia; safer replacements used post-1960s. |
| Ethane | 3 | 15.4 | Chemical precursor to ethylene. |
| Ethylene | 2.7 | 36 | Used for the manufacture of plastics. |
| Gasoline/Petrol | 1.2 | 7.1 | A surprisingly small explosive range! |
| Hydrogen | 4 | 75 | Hydrogen is the simplest atom. |
| Methane | 5 | 17 | Methane is the main component of Natural Gas. |
| Methyl Mercaptan | 3.9 | 21.8 | If skunks made sparks, the smell might be the least of your problems for these potential flamethrowers! |
| Propane | 2.1 | 9.5 | Low carbon-footprint fuel. |
| Silane | 1 | 100 | Pyrophoric gas needs no oxygen. |
| Chlorine Trifluoride | N/A | N/A | Like Oxygen, ClF3 is not combustible but causes anything it contacts to generate flammable by-products. |
Key Facts About The Most Notable Flammable Gases
Ammonia (NH3)
Ammonia is a flammable gas, primarily used to produce fertilizer for crops. It is also employed as a commercial refrigerant due to its cost-effectiveness and efficiency; it is 3-20% more efficient than alternatives, meaning it requires less electricity to produce the same amount of cooling. This makes it ideal for large operations such as ice-skating rinks, food preparation and preservation, and substantial air-conditioning requirements.
This flammable gas is corrosive and toxic to humans if large amounts are released. Its distinctive and powerful odor means leaks will be located quickly with little loss of refrigerant, saving money on replacement costs.
Acetylene (C2H2)
Due to its high explosivity, acetylene cannot be safely compressed beyond 15 psi (1 atmosphere). For this reason, it is stored and transported in cylinders at higher pressures, dissolved in acetone, which stabilizes it.
To prevent the acetylene from separating from the acetone and forming concentrated gas, the cylinders contain a porous material. It is crucial to keep the cylinder upright to prevent acetone from entering the regulator, which could cause safety and quality issues
Butane (C4H10) – Cooking Fuel
Butane is a cheap, colorless, and plentiful flammable gas. Its blue flames are ideal for portable heating needs, such as cooking. It is safe enough for cooking, generating sufficient heat while still having a relatively small energy yield. Butane does not volatilize easily in cold conditions, making it one of the most well-known flammable gases.
Iso-butane, on the other hand, is not used as a cooking fuel but is mainly utilized as a refrigerant. However, iso-butane is also an extremely flammable gas, similar to regular butane. Both butane and iso-butane have four carbon atoms, but they differ in their structural arrangement: butane has a straight-chain structure, while iso-butane has a branched structure.

Carbon Monoxide (CO)
A toxic by-product of incomplete petrochemical combustion, CO is flammable and can even be explosive in concentrations of 12.5 to 74% in air. The toxicity of CO arises from its ability to bind to hemoglobin in red blood cells, preventing them from delivering sufficient oxygen to the body and from releasing the CO effectively.
Exposure to levels as low as 0.03% or 300 PPM can be fatal, which is significantly lower than the concentration required for an explosion.
Hydrogen (H2)
The most common substances in the Universe are hydrogen (capable of powering nuclear reactions) and helium (unless Dark Matter turns out to be real), at about 73% and 25%, respectively of all baryonic matter (made of Protons and Neutrons). Everything else fits into that last 2%, including all of the remaining 116 chemical elements on the Periodic Table.
Given perspective, that is easier to understand—stars are big and planets are small. The Sun is mostly hydrogen and helium, and 1.4 million kilometres wide. Hydrogen powers the nuclear reactions that allow the sun to burn. Earth is just 12,000 km in diameter (less than 1% of the Sun’s width) and it is the densest of all the planets in our Solar System.
The great thing about hydrogen is that this flammable gas is easy to obtain, and burns clean with only water and heat as a by-product. It is one of few flammable gases that doesn’t have a carbon footprint (aside from production costs).
Methane (CH4) – Natural Gas
Natural Gas contains small quantities of ethane, butane, pentane, propane, and even helium, but the vast majority is methane. All of the “‑anes” are odorless, so for safety purposes they are scented with something highly noticeable like ethyl mercaptan or methyl mercaptan so leaks are easily detected. This addition is what gives natural gas its characteristic ‘rotten egg’ smell..
Propane (C3H8)
12,000 people were displaced for months, and even years in some cases, as their homes were rebuilt after a propane depot exploded during an illegal truck-to-truck fuel transfer.
The consequent conflagration was triggered by a Boiling Liquid Expanding Vapour Explosion (BLEVE) when the connections and grounding failed. The company was found guilty of inadequate safety measures and all of its businesses were closed by the government. Amazingly, only two lives were lost.
Propane lends itself to portable fuel needs from cars and buses to tow motor lift trucks, or as a power source in rural areas. This is because it is easy to liquefy at ordinary pressures & temperatures, and retains its ability to become a gas even at low environmental temperatures (unlike butane).
Ethane (C2H6)
This flammable gas is separated in most oil-fields from crude oil, and then cryogenically treated so that the ethane condenses out as a liquid, leaving gaseous methane (et al) behind. It is further distilled to remove propane (et al) until just ethane remains.
Ethane’s chief uses are for manufacturing ethylene for the plastic industry, and steam-cracked to produce ethylene. However, experimental forays into producing Poly-Vinyl Chloride (PVC) are showing some promise with significant cost savings. As well, due to its plentiful supply, vinegar (acetic acid) can be made inexpensively in Saudi Arabia. Some companies are now building engines that can switch between ordinary diesel and ethane fuel.
This flammable gas is also used as a superfast cryogenic coolant to freeze electron microscope samples containing water. Water usually expands when it freezes, rupturing cell walls and causing block-like crystallization that reflects electron beams. Ethane cools it quickly enough to zip past this thermal misadventure, making the samples much more useful.
Ethylene (C2H4)
Ethylene production, to the tune of >50%, is used to make plastics (e.g. polyethylene). This flammable gas is oxidized to produce chemical surfactants used to let detergents release dirt and oils from surfaces (hands, clothes). It is also the main component we use to make ethanol (alcohol) outside of brewing and distilling.
The oxidized ethylene can be made into ethylene glycol for automobile engine antifreeze through hydrolysis. Traditional PVC production occurs through oxychlorination, as does Dry Cleaning Fluid (perchloroethylene), among many others.
This gas evolves naturally, but is also a vital enzyme for the ripening of fruits, triggering branch, shoot, and root growth, and telling leaves when to drop off. However, it can be manufactured more easily than collecting it from natural sources like plants.
In a 2021 discovery, a new enzyme (methylthio-alkane reductase) has been revealed that releases ethylene by scavenging sulfur when it is short of oxygen. This could lead to it becoming a cheap bio-source for the plastic manufacturing industry, reducing the need for petroleum inputs and fossil fuels.

Silane (Silicane) (SiH4)
This pyrophoric (and toxic) gas is used for the deposition of pure silicon in electronic semiconductor manufacturing since it separates from its weak hydrogen bonds relatively easily. However, its pyrophoric nature means that it will experience spontaneous ignition when exposed to air. The dangers are built right in so it requires careful handling at all times.
Japanese manufacturers of solar cells figured out a way to moderate its danger by blending it with hydrogen gas. Their solar cells turned out to be even more stable than before, a free benefit of more thoughtful handling.
Chlorine Trifluoride (ClF3) – The Most Flammable Gas?
While not actually flammable itself, it is composed of two highly reactive elements, Chlorine and Fluorine, that aren’t particularly anxious to be bound together. Think of it like Super-Oxygen, supporting oxidation on a horrific scale able to make just about anything it touches burst into flame. Add it to water, for example, and it will generate chlorine gas and hydrofluoric acid, along with immense heat. If it touches biological materials (e.g. skin) it will burst into vigorous flame, but even plain sand or concrete will burn with ease.
It is so corrosive that it can only be stored in copper, nickel, or alloys of copper-nickel (“Monel”). These metals are safe because they react to form a fluorine-based alloy layer that protects the underlying metal from further interaction. In its gaseous form, it causes nose and airway irritation, lung inflammation, oedema (fluid in the lungs), and exposure represents a serious medical emergency.
Examples of Explosive Gas Atmospheres
As explained earlier, you need three elements to cause a fire or explosion: fuel, oxygen, and a source of ignition. This source of ignition could be sparking electrical equipment or a hot surface with a high temperature.
Let’s delve into real-life scenarios to understand how fires or explosions typically occur.
Methane – House-hold Explosions
Methane leaks are a constant hazard. This gas is lighter than air, preventing it from accumulating at lower levels. However, methane, being a byproduct of bacterial activity in sewage systems, can accumulate underground, posing a significant explosion risk.
Spray Painting Facilities – Industrial Explosions
Industries using solvents must enforce strict safety protocols. For example, spray painting facilities often employ water curtains to trap overspray, funneling it to a reprocessing area. Additionally, in some processes, metal parts are negatively charged, and paint sprayers are positively charged, enhancing paint application efficiency.
Other Potential Sources of Leakage:
- During the process of filling drums, a critical point for leak detection and prevention.
- At flanges within piping systems, where maintaining seal integrity is crucial to avoid leaks.
- Around sight glasses in equipment, as these components can be vulnerable to leakage if not properly maintained or installed.
The petroleum industry, handling a variety of flammable volatiles, necessitates utmost caution. Their operations are largely enclosed, ensuring that most by-products are captured for further processing, such as cracking and distillation, or repurposed for energy generation or as additional feedstock.
Flammable Gases and Safety Data Sheets
It is crucial to thoroughly read the accompanying Safety Data Sheets (SDS) for all flammable gases you work with to prevent fires, explosions, and other hazards. In almost every instance, it is required by law to review the SDSs before interacting with a flammable gas.
This is especially important for new products, as they may have significantly different characteristics compared to products you are more familiar with. The SDS for any specific gas can typically be found on the manufacturer’s or supplier’s website, and also on the NIH website, which is part of the U.S. Department of Health and Human Services.”
The Takeaway
In conclusion, understanding flammable gases is vital in preventing hazardous situations. These gases, which range from common household items like nail polish remover to essential industrial fuels, play a significant role in various applications.
Each type of flammable gas, whether it’s ammonia, butane, or another, has its own unique properties and associated risks. Strict adherence to Safety Data Sheets is crucial in mitigating these risks and avoiding hazardous situations.
By staying informed and exercising caution, we can effectively utilize these gases, ensuring safety and well-being in both personal and professional environments.