Understanding the Minimum Ignition Energy, or MIE, is critical in industries where combustible dusts and vapours are present. For dusts this would include woodworking processes, or metalworking, particularly with polishing and grinding operations; agriculture, as well, since silo explosions can occur when storing grains; and food processing, especially when dealing with flours, sugars, and other extremely fine materials.
Vapours propagate in many working environments, too. Painting operations involving car parts, for example, have an intrinsic danger. Many liquids are volatile but don’t let that oft-confused word mislead you. Water is highly volatile, but since London still exists, we can all agree that fog (water vapour) is not flammable or explosive under most circumstances.
What is MIE in a nutshell?
Minimum Ignition Energy (MIE) is the lowest energy required to ignite a mixture of a flammable substance with air, measured in millijoules (mJ).
It varies with the type of substance and its concentration and is critical for assessing explosion risks in safety applications.

Industrial Processes Prone to Explosions
The danger lies in combustible or flammable vapours, like VOCs (Volatile Organic Chemicals) which include hydrocarbons. This name may partially explain why some people confuse volatile with explosive. VOCs also include alcohols, aldehydes, ketones, ethers, and aromatics like benzene, all of which are flammable.
Consequently, danger exists in petroleum processing, oil and gas exploration, and doubly so in coal mining operations where both dust and flammable vapours (methane) coexist.
Other dangerous areas include things you wouldn’t suspect, like pill manufacturing where powders are highly compressed to make tablets—indeed, even preparing the powders for that purpose. And don’t forget rubber and plastic manufacturing, using powders in the process, and generating more in finishing operations.

Equally unexpected might be the textile industry where bolts of cloth are unwound and cut to size at high speed, launching fibres into the air, and creating static discharges in the process. The same applies to the paper and printing industry, and for precisely the same reasons.
Combined industries, like recycling, can face all of these dangers at once when recycling metals, oils, fuels, paper, and plastics. This is why proper precautions and safety measures, including effective dust control, static discharge technology, and explosion prevention strategies, are essential to minimize the risk of dust or vapour related fires and explosions. Surprisingly the biggest risk is found inside the combustible dust collection systems due to overlooked maintenance!
How Do We Measure MIE?
Typically we measure MIE in a laboratory setting with gear designed for the purpose. The two most common methods involve either the Direct Spark method (sustained or static), and the Hot Surface method.
In the first method, a test substance is evenly distributed in a chamber, and a low energy spark is introduced. In the case of dust, a mechanical system, like an air cannon will launch the material into a uniform cloud. Flammable vapours are much easier to work with obviously. The energy of the spark is increased until ignition is obtained and that value becomes the MIE, but only for that specific sample. Testing is usually done under STP (Standard Temperature and Pressure) conditions and then extrapolated for field conditions..
The Hot Surface method uses a heated wire or coil and the temperature is raised until ignition is achieved. That is recorded as the MIE for that dust or gas.
Approriate units for measuring MIE
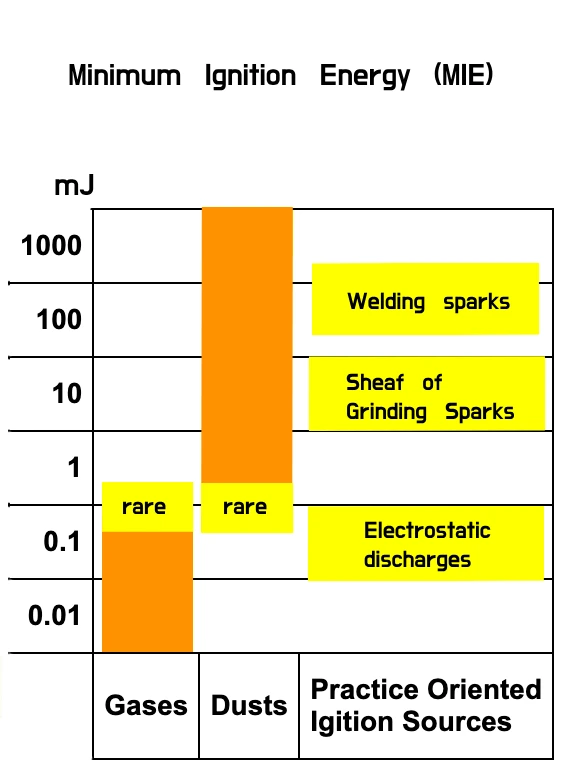
MIE is typically measured in units of energy per unit mass, such as millijoules per gram (mJ/g) or volumetrically as microjoules per cubic meter (µJ/m³). The lower the MIE value, the easier it is for the fuel to ignite. Anything possessing an MIE <3 mJ is extremely sensitive to ignition and would demand very precise handling measures. By way of example, hydrogen in air at a 30% concentration would ignite at just 0.0057 mJ, or 5700 µJ of energy.
[Table] Mie of various substances
| Dust material | MIE (mJ) |
|---|---|
| Aluminium | <1 |
| Bio-waste | >1000 |
| Coal | >1000 and 60 |
| Anthracite | 100 |
| Bituminous coal | 30 |
| Lignite | 30 |
| Charcoal | 20 |
| Magnesium | >1000 and 80 |
| Zinc | 300 and 9600 |
| Polystyrene | 100 |
| Urea | 100 |
| Calcium stearate | 10 |
| Cornstarch | 10 and 30 |
| Corn flour | 20 and 40 |
| Pea flour | 40 and 100 |
| Sawdust | 10 |
| Sugar | 10 and 30 |
| Sewage sludge | 100 and 8000 |
| Sulphur | <1 |
| Yeast | 100 |
| Wood | 40 |
What Influences Its Explosivity?
Particles bigger than 0.5 mm are resistant to ignition—less than that size increases the ignition danger.
Combustible dust
For combustible dusts, the MIE depends on various factors, including the particle size distribution, concentration in the air, and the specific properties of the dust itself. Finer dust particles have a higher surface area per unit mass, and are more prone to ignition. Additionally, the presence of certain gases in the environment can also influence the MIE. Even temperature can have a strong influence.
For example, a small shallow dish of kerosene will extinguish a lit match at room temperature, but if the kerosene is heated to 45℃ it will ignite because the vapour pressure is high enough for the match flame to supply the MIE. In this case, the “fuel” component of the fire triangle is missing until heat is applied to the kerosene.
Explosive gases and vapors
This is also the explanation for why gas tanks never explode in cars (except in Hollywood Movies) because the fuel density is too high and there is insufficient oxygen available. As always, you can still get a fire, of course, when the fuel and vapour acquire sufficient oxygen from leaks or spillage. Interestingly, those stunt explosions are achieved with something called Lycopodium powder specifically because it is a very fine dust which ignites easily. It is launched into the air with an air-canon through a spark to make magnificent but short-lived fireballs that are extremely safe when handled by pyro-technicians.
Decreasing explosivity
You can decrease explosivity by adding inerting substances such as sodium bicarbonate (used in fire extinguishers, since it is non-flammable and non-combustible). The relationship between v/v% (volume per volume) or w/w% (weight per weight) presence of inerting substances is not linear. Something with up to 80% inerting substance may still be significantly ignitable.
MIE varies more closely with the inerting component, but is defined by the finest fraction of the combustible substance. If the particles become finer due to sieving and transfer operations during processing, the MIE can plummet presenting a hazard in (for example) pharmaceutical manufacturing.
The Takeaway
Minimum Ignition Energy is not an intrinsic property of a substance or material. It is specific to the individual sample and its size distribution, usually based on the mean size, but the smallest particle size in the sample can significantly affect the MIE. Under ATEX, MIE plays a critical role in identifying hazards.
It is also based on the morphology or shape of the particles, since irregular particles may present more surface area compared to volume, and decrease MIE. As an analogy, it would be much easier to ignite a slice of bacon than a cube of meat with the same mass since the surface area is comparatively huge. MIE varies, too, with moisture content which inhibits ignition, and higher humidity during processing can decrease the chances of sparks (electrostatic discharge).
The atmospheric pressure can change how flame fronts propagate through a gas or dust cloud. Ambient temperature affects the MIE, as does the oxygen level. In fact, the Minimum Oxygen Concentration (MOC) is closely linked to the energy of the ignition source, with a direct correlation observed: higher oxygen concentrations reduce the requirement for ignition energy, while lower concentrations increase the need.
In most cases, standardized testing procedures are available through organisations such as the American Society for Testing and Materials (ASTM) or the International Electrotechnical Commission (IEC) to provide guidelines for testing MIE.
MIE varies, even with the same material, depending on many external conditions. Extrapolating from laboratory testing results can be useful, but getting specific tests for your samples and conditions is far more revealing.



