Cobic-Ex will explain everything you need to know about the lower explosive limit in this article. We will also explore how the LEL varies among different substances and discuss additional topics related to the lower explosion limit.
What Is LEL?
For an explosion to occur, three elements must be present: air, combustible gas, and an ignition source. These elements are also known as the fire triangle. However, for the combustible gas to be capable of causing an explosion, it must be within a specific concentration range. This is where the concepts of the lower and upper explosive limits become important. Consequently, this leads to the question: What does LEL mean?
The lower explosive limit represents the minimum concentration of a specific gas or vapor required to burn in air. This limit is expressed as a percentage and varies between gases and vapors. For example, the LEL of ethane is approximately 3%, while that of methane is 5%. This indicates that a lower concentration of ethane, compared to methane, is needed to support ignition.
From this understanding, we can deduce that the concentration range necessary for ignition varies depending on the substance. This brings us to the significance of the upper explosive limit (UEL), which will be discussed in the following section

The Lower And Upper Explosive Limits
In addition to requiring a minimum concentration to support ignition, gases, and vapors also have a maximum concentration threshold beyond which they cannot contribute to an explosion. The upper explosive limit (UEL) represents this threshold: it is the highest concentration of a gas or vapor in the atmosphere that can produce a flash or fire when an ignition source is present.
But what happens if the concentration of a particular flammable gas falls below the lower explosive limit or exceeds the upper explosive limit in the presence of an ignition source? Interestingly, the answer is nothing. In these situations, the concentration of the flammable gas is not within the range necessary to generate a flash or fire.
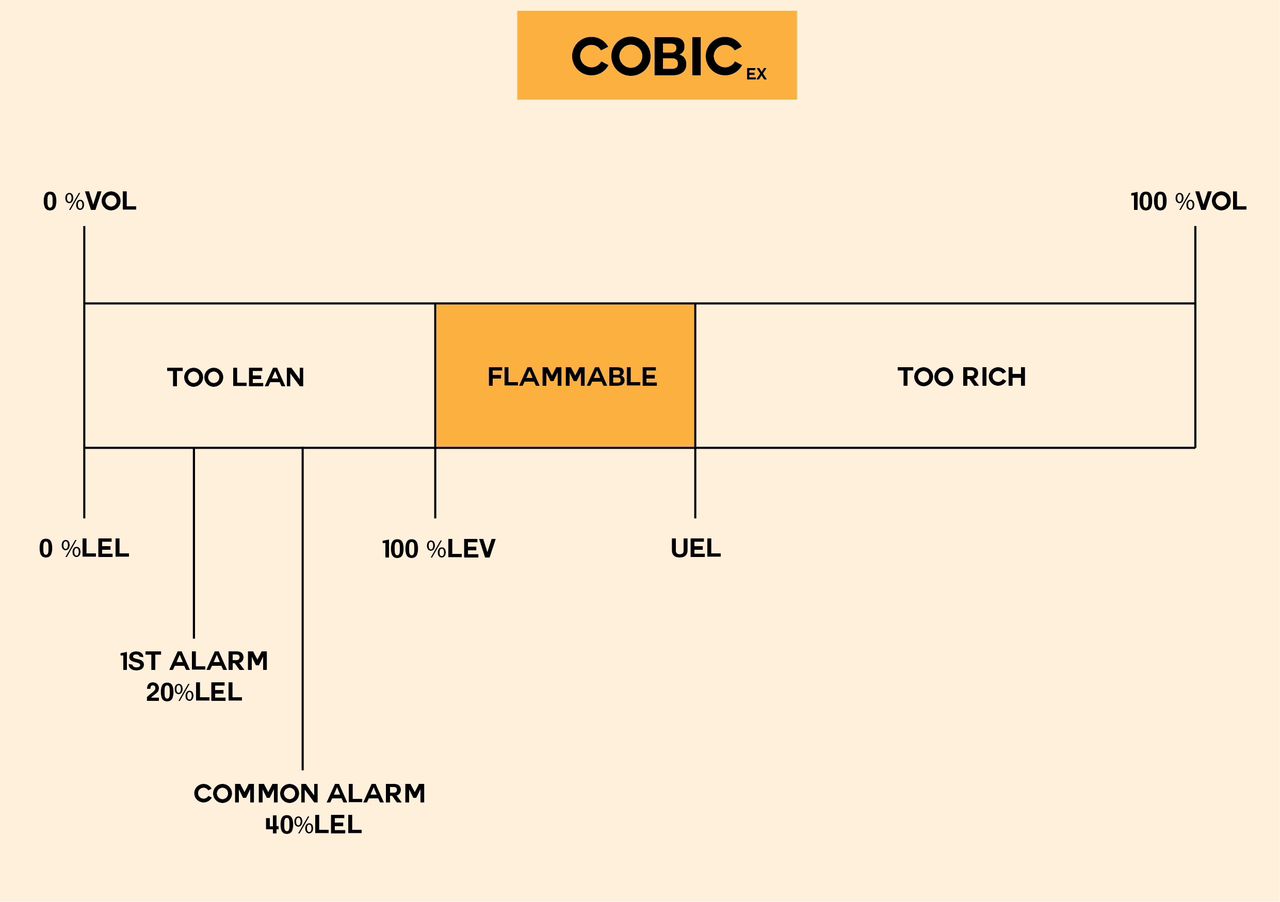
In the image above, the LEL and UEL are graphically represented to facilitate a better understanding of these concepts. Additionally, in terms of gas/air mixtures, a mixture is termed ‘too rich’ if its concentration surpasses the upper explosive limit. Conversely, if the concentration of a flammable gas falls below the lower explosive limit, the gas/air mixture is considered ‘too lean.’
The Lower Explosive Limit of Various Substances
Questions such as ‘What is the gas LEL?’, ‘What is the LEL of methane?’, and ‘What is the LEL of hydrogen?’ are commonly asked in contexts dealing with flammable gases and vapors. Environments that contain combustible gases and vapors are typically classified as ATEX Zones.
To address these queries, we have included a table that illustrates the lower explosive limit (LEL) and upper explosive limit (UEL) of various flammable gases and vapors, measured in volume.
| Type of gas | LEL | UEL |
| n-Butane | 1.8% | 8.4% |
| Iso-Butane | 1.8% | 8.4% |
| Ethane | 3% | 12.4% |
| Ethylene | 2.7% | 36% |
| Hexane | 1.2% | 7.4% |
| Hydrogen | 4% | 75% |
| Methane | 5% | 17% |
| Pentane | 1.5% | 7.8% |
| Propane | 2.2% | 9.5% |
It is noteworthy that hydrogen has a considerably broad range within which it can produce a flash or fire when exposed to an ignition source, necessitating cautious handling of this substance.
In contrast, pentane’s explosive limits are much closer together, resulting in a narrower range that supports ignition.
Consequently, compared to hydrogen, pentane poses a lower risk in environments where explosions are a potential hazard.
How Is The Lower Explosive Limit Measured?
Flammable gases and vapors are common in various industries, including oil and gas, chemical production, and transportation. Ensuring worker safety in these explosive atmospheres is paramount. This involves monitoring the concentration of these gases to maintain safe conditions where flammable gases and potential ignition sources coexist. But how is this concentration measured?
Typically, the concentration of gases in an area is determined using sensors, often referred to as ‘LEL sensors.’ These sensors measure the difference in resistance between two elements, one of which contains a catalyst. This resistance correlates to the lower explosive limit.
However, there are various methods to measure the concentration of certain gases within an area. It’s important to note that safety is not automatically ensured by the use of LEL sensors alone. Therefore, it’s crucial to implement comprehensive safety measures in your work environment.
For any questions or additional information, please don’t hesitate to reach out to us at info@cobic-ex.com. And remember, explosive atmospheres are more common than you might think!





